SOURCE: John Campbell, YouTube, 31 Dec. 2022
REVIEW
In a YouTube video uploaded on 31 December 2022, retired nurse practitioner John Campbell discussed vaccine adverse events following COVID-19 vaccination. Citing the manner in which previous vaccine safety concerns—namely the swine flu vaccine used in the U.S. in 1976 and the Rotashield vaccine—were handled, Campbell presented the rate of serious adverse events following COVID-19 vaccination reported in a published study, which was higher than the swine flu vaccine and the Rotashield vaccine.
He concluded by saying that the COVID-19 vaccines were “officially promoted”, in contrast to the other two, adding that “You’ll be as confused as I am” over this state of affairs. In a tweet by Campbell sharing the video, the gist of his video was made more clearly: “Serious adverse vaccine events, 1 in 800. Reanalysis of data from original phase III trials. This is too high”.
The video received more than 830,000 views, and was shared more than 6,000 times on Facebook, according to social media analytics tool CrowdTangle. Campbell’s tweet received more than 6,500 engagements, including more than 1,700 retweets.
The rate of serious adverse events for COVID-19 vaccines that Campbell cited originates from a study published in September 2022 in the scientific journal Vaccine[1].
In brief, the study compared adverse events following COVID-19 vaccination with the risk of COVID-19 hospitalization, in order to estimate the harm-benefit ratio of COVID-19 vaccination. The authors reported that “Pfizer and Moderna mRNA COVID-19 vaccines were associated with an excess risk of serious adverse events of special interest of 10.1 and 15.1 per 10,000 vaccinated over placebo baselines of 17.6 and 42.2” and concluded that “These results raise concerns that mRNA vaccines are associated with more harm than initially estimated at the time of emergency authorization”.
It’s not the first time that this study was used to suggest that the COVID-19 vaccines are unsafe; Health Feedback previously reported on the misleading coverage of the study, then a preprint, in an earlier review. Other scientists criticized the flawed methodology of the study cited by Campbell months before the video was published, but none of these are mentioned in the video.
Campbell previously made videos containing misinformation about COVID-19 and vaccines; Health Feedback and others covered his claims here and here.
This review will summarize some of the important flaws in the study. It will also show why the comparison made by Campbell between the COVID-19 vaccines and historical vaccines is misleading, as it presents a one-sided view of the reasons behind the withdrawal of the swine flu vaccine and Rotashield vaccine.
The study analyzed data on serious adverse events (SAEs) that occurred during Pfizer and Moderna’s Phase 3 clinical trials in adults. The safety data in the Pfizer trial had a data cut-off date of 9 October 2020, according to the published results of the trial in the New England Journal of Medicine[2], while those of the Moderna vaccine trial had a data cut-off date of 25 November 2020[3]. The time period in which these SAEs occurred spanned a median of two months.
The authors matched these SAEs to a list of adverse events of special interest (AESIs), defined by the Brighton Collaboration, a non-profit that seeks to improve vaccine safety. The number of AESIs were used as a measure of vaccine harm. COVID-19 hospitalizations that occurred in the same period were used to measure vaccine benefits.
However, experts pointed out several gaps in the methods used to estimate the benefits and harms of vaccination in the study.
David Spiegelhalter, chair of the Winton Centre for Risk and Evidence Communication at Cambridge University, told Full Fact that the study considered only COVID-19 hospitalization as the basis for measuring benefit from vaccination, and that this was only for two months and at a time when COVID-19 cases were relatively low.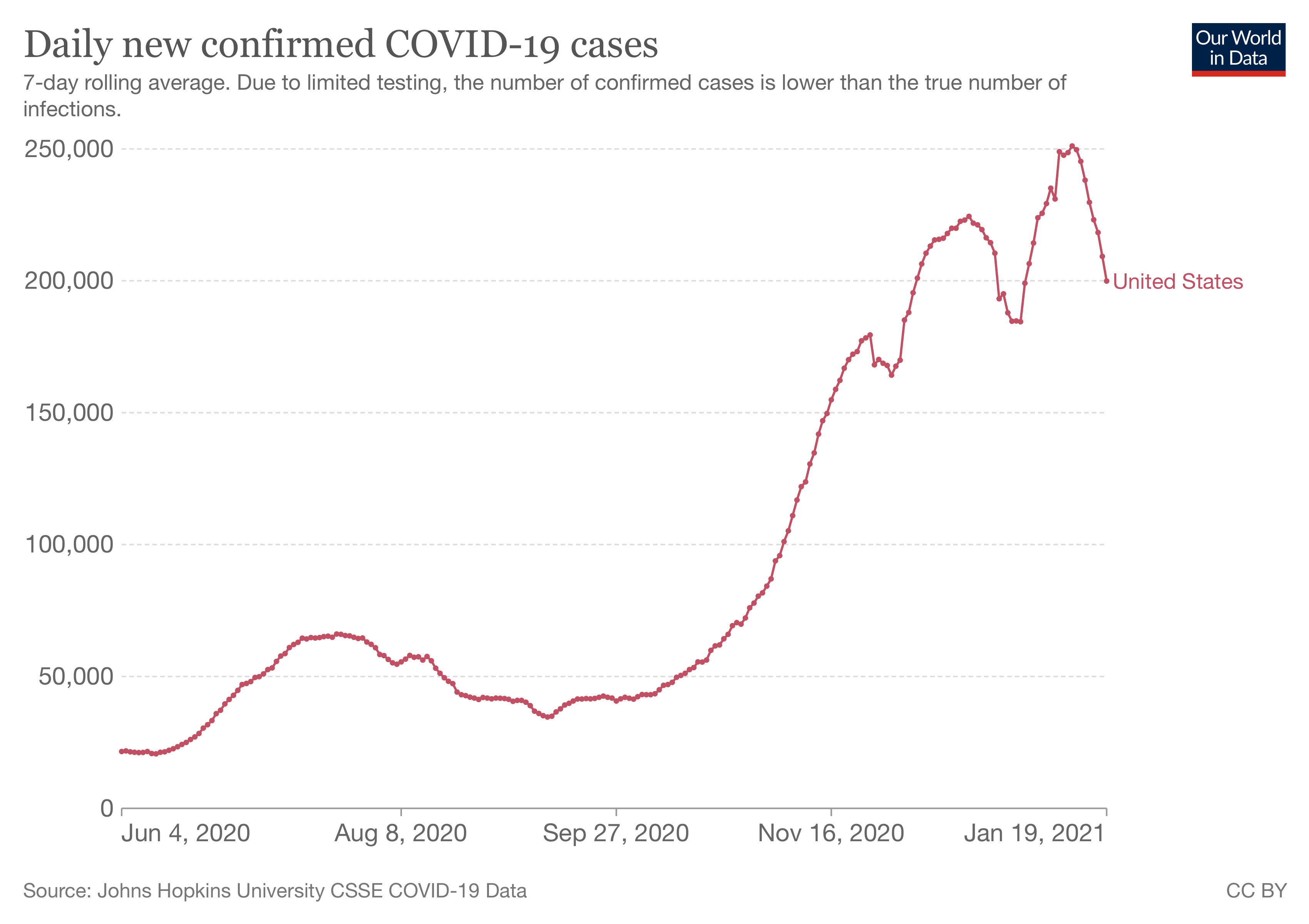
Figure 1. The daily number of confirmed COVID-19 cases in the U.S. Source: Our World in Data.
“The true benefit of vaccines extend far beyond this period, so the harm/benefit comparison used in this study seems entirely inappropriate—the trials were not designed to estimate the reduction in severe Covid,” he said.
In contrast, harms from vaccination usually show up within the first six weeks. Therefore, while the relatively short duration used by the study would have been sufficient to pick up most vaccine harms, if not all, the same isn’t true of vaccine benefits.
Neurologist Jonathan Howard, who writes for the blog Science-Based Medicine, also pointed out the same issue in this article:
“First, depending on how quickly a virus spreads, the benefits of a vaccine can take many months, even decades to accumulate. Second, nearly all vaccine harms occur right after vaccination. Someone who analyzed the RCTs after one week would find the vaccine caused a bunch of people to feel unwell, while preventing zero cases of COVID. In all vaccine RCTs, the harms are frontloaded, while the benefits accrue gradually over time.”
To illustrate how it takes more time for vaccine benefits to become evident compared to vaccine harms, Howard cited a graph in the published study on Pfizer’s Phase 3 clinical trials[2], showing the cumulative number of COVID-19 cases in the placebo group and the vaccinated group over time.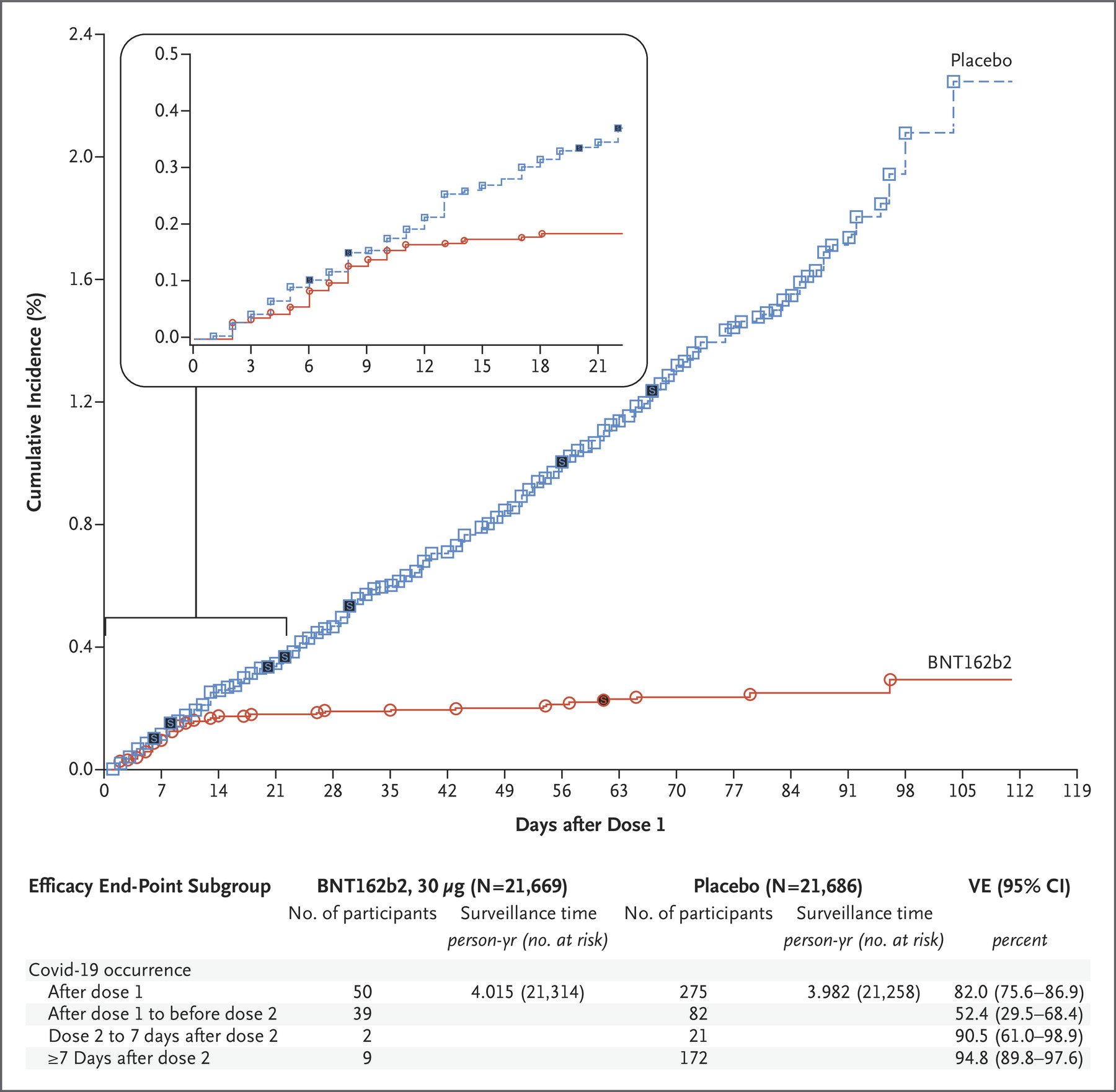
Figure 2. The cumulative number of COVID-19 cases in the placebo group and the vaccinated group, any time after the first dose of the Pfizer-BioNTech COVID-19 vaccine, expressed as a percentage[2].
Another issue is the fact that COVID-19 hospitalizations could only be counted once, in the context of the relatively short duration studied, but AESIs were counted on a per-event basis. Jeffrey Morris, professor of biostatistics at the University of Pennsylvania, called this a “glaring methodological flaw” in a Twitter thread discussing the study.
In the authors’ response to Full Fact regarding this criticism, which can be read in full on Zenodo, they wrote that this had to be done because they didn’t have access to individual-level data. Regardless of the reasons, the fact remains that this manner of comparison biased the analysis towards counting a greater number of AESIs compared to COVID-19 hospitalization.
Finally, the included AESIs—the chosen measure of vaccine harms—don’t always compare to COVID-19 hospitalization in terms of severity. In her YouTube video about the study, Susan Oliver, a nanomedicine scientist at the University of New South Wales, pointed out that conditions like rash, diarrhea, and arthritis, while defined as AESIs, don’t typically require hospitalization.
In other words, claims like Campbell’s draw a false equivalence between the measured vaccine harms and COVID-19 hospitalization. Oliver called this comparison one of “apples and oranges”.
In summary, because of the methods used, the study doesn’t provide reliable evidence for Campbell’s suggestion that the risk of vaccine-related adverse events is greater than that of COVID-19. However, none of the problems with the study’s methods, which were pointed out a few months before the video was published, were acknowledged by Campbell.
In the video, Campbell reached back into history to discuss how previous vaccine safety concerns were addressed, citing the adverse events associated with the 1976 swine flu vaccination campaign in the U.S. and the Rotashield vaccine as the cause of both vaccines’ withdrawal from the market. Campbell contrasted the rate of adverse events for these two vaccines with the higher one cited for COVID-19 vaccines, expressing puzzlement over why the COVID-19 vaccines are “officially promoted” while the other two were withdrawn.
In truth, the motivations for withdrawing both vaccines were more complex than that presented by Campbell.
The 1976 swine flu vaccination campaign in the U.S. began after a flu outbreak at Fort Dix in New Jersey. Scientists had discovered that the flu virus responsible for the outbreak appeared to be genetically similar to the one that caused the deadly 1918-1920 flu pandemic, which led to an estimated 25 to 50 million deaths worldwide.
Fearing a repeat of this pandemic, the U.S. government fast-tracked the development of a flu vaccine and promoted its uptake in a national flu vaccination campaign. But the campaign soon ran into problems, as recounted by modern reports in the BBC, the Smithsonian Magazine, and Discover Magazine.
One of which was the perceived safety problems with vaccines. At that time, vaccine manufacturers sought liability protection, which insurers refused to give. A story about three elderly people who experienced a heart attack after getting the flu vaccine also emerged, further compounding the belief that there was something wrong with the vaccine.
More importantly, the fears over a deadly swine flu pandemic had turned out to be overblown, as there wasn’t widespread transmission of the virus in the end.
Finally, it later emerged that the vaccine was associated with a slight increase in Guillain-Barré syndrome (GBS), an autoimmune disorder affecting the nerves. The risk was estimated at about 1 per 100,000 recipients. GBS commonly develops after an infection; in fact, getting the flu itself is also associated with an increased risk of GBS.
In a 2009 interview for the Bulletin of the World Health Organization, Harvey Fineberg, then president of the U.S. Institute of Medicine, stated that these cases of GBS “wouldn’t have been a blip on the screen had there been a pandemic but, in the absence of any swine flu disease, these rare events were sufficient to end the programme”.
An article published in the journal Emerging Infectious Diseases also highlighted the fact that the withdrawal involved weighing up both the risks and the benefits:
“Had H1N1 influenza been transmitted at that time, the small apparent risk of GBS from immunization would have been eclipsed by the obvious immediate benefit of vaccine-induced protection against swine flu. However, in December 1976, with >40 million persons immunized and no evidence of H1N1 transmission, federal health officials decided that the possibility of an association of GBS with the vaccine, however small, necessitated stopping immunization, at least until the issue could be explored.” [emphasis added]
Therefore, the decision to withdraw the vaccine wasn’t solely due to the number of adverse events observed, but also because the risk posed by the disease in this case was so small that the risks of vaccination weren’t justified by the benefits.
The same story can be told with the Rotashield vaccine, which protects against severe rotavirus disease. Rotavirus is the most common cause of severe diarrhea in children and infants worldwide. A 2018 study in JAMA Pediatrics estimated that rotavirus caused more than 120,000 deaths in 2016, with more than 80% of these deaths occurring in sub-Saharan Africa[4].
The Rotashield vaccine was withdrawn less than a year after licensure, after scientists discovered that the vaccine was associated with an increased risk of intussusception in children younger than 12 months. Intussusception is a serious but treatable condition in which the intestine folds in on itself like a telescope, causing bowel obstruction. According to the U.S. Centers for Disease Control and Prevention (CDC):
“The risk of intussusception increased 20 to 30 times over the expected risk for children of this age group within 2 weeks following the first dose of RotaShield® vaccine. The risk increased 3 to 7 times over the expected risk for this age group within two weeks after the second dose of RotaShield® vaccine.”
This discovery led the Advisory Committee on Immunization Practices to withdraw its recommendation for the Rotashield vaccine and the manufacturer voluntarily withdrew the vaccine from the market in October 1999. No rotavirus vaccine became available until 2006.
It’s important to note that the ACIP decision to withdraw the vaccine was based on the severity of intussusception and “because of the perception that most of the severe complications of rotavirus gastroenteritis in the United States can be prevented by oral rehydration”, according to the CDC. In other words, the severe effects of rotavirus were easier to mitigate in the U.S. Consequently, the risk posed by the disease wasn’t considered to be greater than the risk posed by the vaccine’s side effect.
In a 2012 article for the Milbank Quarterly, bioethics expert Jason Schwartz reported that the decision by the ACIP to cease recommending Rotashield in the U.S. ultimately discouraged other countries, including developing nations that faced a greater risk from rotavirus than the U.S., from using the vaccine[5].
In summary, the decisions to withdraw the 1976 swine flu vaccine and the Rotashield vaccine were based on multiple considerations, contrary to the simplistic picture painted by Campbell. While the safety issues played an important role, they were also considered in the context of the risks posed by the disease, which aren’t static; risks can change over time like virus transmission or may differ depending on a country’s socioeconomic status.
Campbell’s suggestion that the rate of adverse events following vaccination alone was the basis for the withdrawal of the two vaccines—which by extension suggests that these numbers alone serve as a valid measure of vaccine safety—is misleading for viewers.
Finally, another problem with Campbell’s comparison is that the numbers for the 1976 swine flu vaccine and the Rotashield vaccine were specific to one particular adverse event known to be associated with the vaccines—GBS and intussusception, respectively—and therefore are expected to be fairly small. The adverse events reported for the COVID-19 vaccines, on the other hand, comprised many different adverse events, including diarrhea and arthritis, neither of which are associated with the COVID-19 vaccines. In short, this is once again a case of comparing apples and oranges.
COVID-19 Vaccine
Published on: 06 Jan 2023 | Editor: Flora Teoh
Health Feedback is a non-partisan, non-profit organization dedicated to science education. Our reviews are crowdsourced directly from a community of scientists with relevant expertise. We strive to explain whether and why information is or is not consistent with the science and to help readers know which news to trust.
Please get in touch if you have any comment or think there is an important claim or article that would need to be reviewed.
Get email news updates:
Follow us:
See how we rate claims
We depend on your support to operate. Help us create a more trustworthy Internet!
Donate
Reviewers
Community standards
Apply to become a reviewer
About
Our method to evaluate articles
Our method to evaluate claims
Contact us
